What Is The Charge On A Proton?
Charge Of A Proton: A proton is a subatomic particle with a positive electric charge. Protons are in every atom’s nucleus. In fact, the number of protons in each atom is its atomic number.
The proton is now considered a fundamental particle. However, new technologies have led to the discovery that the proton is actually composed of smaller particles called quarks. A quark is a fundamental particle of matter that only recently has been discovered. There are six known types of quarks: up, down, strange, charm, top, and bottom. A proton is composed of two up quarks and one down quark.
A proton can be formed as the result of an unstable neutron. After about 900 seconds away from a nucleus, a neutron will break down radioactively into a proton, electron, and an anti-neutrino. Unlike a neutron, a free proton is stable. When free protons interact with each other, they form hydrogen molecules. Our sun, along with most other stars in the universe, is primarily composed of hydrogen.
Charge Of A Proton In Coulombs
Protons, together with electrically neutral particles called neutrons, make up all atomic nuclei except for the hydrogen nucleus (which consists of a single proton). Every nucleus of a given chemical element has the same number of protons. This number defines the atomic number of an element and determines the position of the element in the periodic table. When the number of protons in a nucleus equals the number of electrons orbiting the nucleus, the atom is electrically neutral.
The discovery of the proton dates to the earliest investigations of atomic structure. While studying streams of ionized gaseous atoms and molecules from which electrons had been stripped, Wilhelm Wien (1898) and J.J. Thomson (1910) identified a positive particle equal in mass to the hydrogen atom. Ernest Rutherford showed (1919) that nitrogen under alpha-particle bombardment ejects what appears to be hydrogen nuclei. By 1920 he had accepted the hydrogen nucleus as an elementary particle, naming it proton.
One or more protons are present in the nucleus of every atom; they are a necessary part of the nucleus. The number of protons in the nucleus is the defining property of an element and is referred to as the atomic number (represented by the symbol Z). Since each element has a unique number of protons, each element has its own unique atomic number.
The word proton is Greek for “first”, and this name was given to the hydrogen nucleus by Ernest Rutherford in 1920. In previous years, Rutherford had discovered that the hydrogen nucleus (known to be the lightest nucleus) could be extracted from the nuclei of nitrogen by atomic collisions. Protons were therefore a candidate to be a fundamental particle, and hence a building block of nitrogen and all other heavier atomic nuclei.
Where is the proton located?
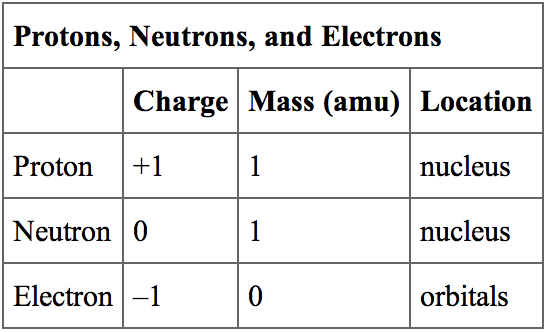
Protons and neutrons are found in the nucleus. They group together in the center of the atom.
What is the proton symbol?
| Particle | Symbol | Mass |
|---|---|---|
| electron | e– | 0.0005486 amu |
| proton | p+ | 1.007276 amu |
| neutron | no | 1.008665 amu |
How big is a proton?
What Is The Charge Of A Proton
High-energy particle-physics studies in the late 20th century refined the structural understanding of the nature of the proton within the group of subatomic particles.
Protons from ionized hydrogen are given high velocities in particle accelerators and are commonly used as projectiles to produce and study nuclear reactions. Protons are the chief constituent of primary cosmic rays and are among the products of some types of artificial nuclear reactions.
Read Also: The Doppler Effect Equation
Although protons were originally considered fundamental or elementary particles, in the modern Standard Model of particle physics, protons are classified as hadrons, like neutrons, the other nucleon (particles present in atomic nuclei), composite particles composed of three valence quarks: two up quarks of charge +2/3e and one down quark of charge –1/3e. The rest masses of quarks contribute only about 1% of a proton’s mass, however. The remainder of a proton’s mass is due to quantum chromodynamics binding energy, which includes the kinetic energy of the quarks and the energy of the gluon fields that bind the quarks together. Because protons are not fundamental particles, they possess a measurable size; the root means square charge radius of a proton is about 0.84–0.87 FM or 0.84×10−15 to 0.87×10−15 m.
Charge Of A Proton And Electron
Atoms were once thought to be the smallest building blocks of the universe until it was discovered that even they were constructed of building blocks of their own. Those building blocks are protons, electrons, and neutrons, and with the advancement of science, it has been discovered that each of these has its own unique properties as well.
Mass
The mass of an individual proton is 1.672621636(83)í–10 (-27) kg. The collective mass of the protons in the nucleus of an atom is roughly the same as the mass of all the neutrons. Of all the weight of an atom, more than 99 percent of the mass is contained in the nucleus; therefore, nearly half of the mass of the atom is made up of protons. The mass of a proton is approximately 1,860 times more than the mass of an electron.
Charge
The charge of the proton is a positive charge. The nucleus of the atom is made up of positively charged protons and negatively charged neutrons. The positive charge that is carried by the proton is called a +1 elementary charge, the exact opposite of the negative charge that is carried by a single electron. It is called an elementary charge because it is theoretically the smallest charge possible. (This has since been proven wrong with two exceptions–the quark and the quasiparticle). One thing that has never been proven wrong, however, is that the charge is a constant. Regardless of circumstances including things like temperature, pressure, and even time, the elementary charge of a proton will not change.
